Tokyo University of Pharmacy and Life Sciences, Department of Applied Life Sciences, Lab. Bioengineering
Tel: +81-42-676-7141 Fax: +81-42-676-7145
Learn from life, and design life
Professor: Kazuma TOMIZUKA, Ph.D.
Homepage
Associate Professor: Masatada TAMAKOSHI, Ph.D.
Homepage
Lecturer: Shin-ichi YOKOBORI, Ph.D.
Homepage
Under construction
The research of ancestral genes that were possessed by the last universal common ancestor "Commonote"
We are trying to analyze the last universal common ancestor that lived on the Earth billions year ago. We can resurrect, reconstruct and analyze the genes of the ancestral organism, using the genetic information of various extant organisms.
The evolutionary study of invertebrates
We are studying molecular evolution of invertebrate animals. Many aspects of evolution of non-fossilized organisms remain to be elucidated. We are investigating the evolution of invertebrates by the analyses of their mitochondrial genomes.
Tanpopo mission
Living organisms might have moved from the earth to other planets. To investigate the panspermia hypothesis and the possible space origin of organic compounds, we performed space experiments at the Exposed Facility (EF) of the Japanese Experiment Module (JEM) of the International Space Station (ISS). We capture any orbiting microparticles, such as micrometeorites, space debris, and terrestrial particles carrying microbes as bioaerosols, by using blocks of silica aerogel.
The research on protein folding and stability
A goal of our laboratory is to solve the folding mechanism of proteins. To this end, we are trying to identify the protein folding pathway and the independent folding units within a protein structure by means of experimental and computational methods. We are also analyzing the thermodynamic stability of proteins.
Study of biological macromolecular complex
We are trying to elucidate the structure of macromolecular complex: pili of thermophiles and thermophilic phage particles.
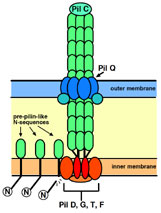
A proposed model for pilus biogenesis and extension
Modification of enzymes by evolutionary engineering
Enzymes are biocatalyst that can discriminate even enantiomers. In order to apply enzymes for industrial purposes, we are developing evolutionary molecule engineering techniques to improve several properties of thermophilic enzymes: adaptation of the enzymatic activities to low temperatures and conversion of substrate specificity. To date, we have succeeded in improving the catalytic activity of thermophilic enzymes at low temperatures without loss of thermostability
Bio-nanotechnology
The Japanese government has selected four major scientific research targets for the 21st century. One is life science, and another is nanotechnology. Because thermophilic proteins are quite robust, they are potentially suitable materials for bio-nanotechnology. We are constructing protein nano-blocks from thermally stable proteins. The final goal of this project is to apply the designed proteins to nano-mechanics and nano-electronics.
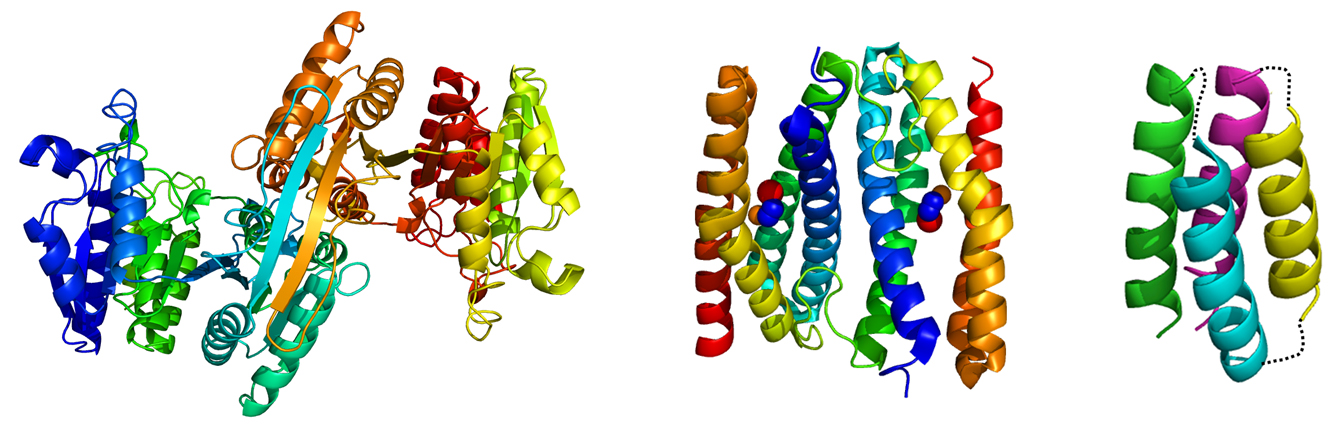
Thermally stable native and artificial proteins used in bio-nanotechnological studies
Copyright(c)2005-2017 Lab. Bioengieering., Dept. Appl. Life Sci., Tokyo Univ. Pharm. Life Sci. all right reserved.


